Overview
The vertebrate musculoskeletal system is essential for structural support and locomotion. It is composed of muscle which is surrounded by muscle connective tissue and attached via tendons to bone. The broad aim of our laboratory is to understand the molecular mechanisms and cellular interactions regulating musculoskeletal development, regeneration, disease, and evolution. Our research is centered on two components of the musculoskeletal system: muscle and muscle connective tissue. We focus on the muscle stem cells because they are required for generating muscle (which is post-mitotic) during development, growth, and regeneration. We focus on the connective tissue because not only is it critical for musculoskeletal form and function, but it provides the molecular and cellular niche within which muscle stem cells reside. The goal of our research is to elucidate the role of muscle stem cells, connective tissue, and muscle/connective tissue interactions during development of the limb and diaphragm, muscle regeneration, and musculoskeletal evolution.
Muscle Stem Cells in Development, Regeneration, and Homeostasis
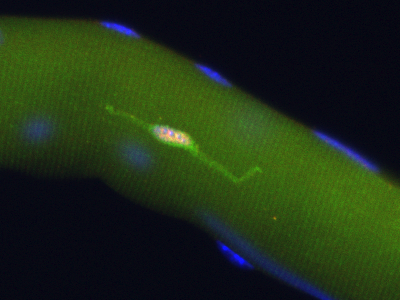 |
Pax7+ (pink nucleus) satellite cell is genetically labeled with YFP in Pax7CreERT2/+;R26RYFP/+ mice |
Muscle development, growth, and regeneration take place throughout vertebrate life. All of these processes are mediated by muscle stem cells. Our research has concentrated on elucidating the developmental origin of muscle stem cells in limb muscle, their function, and the extrinsic signals that regulate them. Although widely recognized that embryonic, fetal/neonatal, and adult muscle are distinct, it was unclear whether they derived from a single or multiple stem cell populations. In a series of lineage experiments (Kardon et al. 2002, Schienda et al. 2006, Hutcheson et al. 2009), we determined that all stem cells originate from the somites, but embryonic and fetal muscle derive from developmentally distinct, although related stem cells. In addition, local limb signals, likely from nearby connective tissue fibroblasts, significantly regulate muscle development. Currently, we are determining what are these signals, testing where they come from, and determining how these signals are regulating muscle stem cells and their development.
Are muscle stem cells necessary for muscle development, regeneration, and homeostasis? To test this, we have genetically ablated different stem cell populations. Genetic ablation of stem cells expressing the transcription factors Pax3 or Pax7, demonstrated that Pax3+ and Pax7+ stem cells are required for embryonic and fetal myogenesis, respectively (Hutcheson et al. 2009). In the adult, muscle satellite cells have long been proposed to be the stem cell required for muscle regeneration, and many studies have demonstrated that satellite cells are sufficient to regenerate muscle. However, the necessity of satellite cells had not been critically tested. Using a tamoxifen-inducible Pax7CreERT2 allele, which we generated to genetically manipulate satellite cells with high efficiency (and now available at Jax), we definitively demonstrated that satellite cells are the endogenous stem cell required for muscle regeneration (Murphy et al. 2011). Similar results were also found by our colleagues (Lepper et al. 2011, Sambasivan et al. 2011, McCarthy et al. 2011). Thus, satellite cells are the key stem cell required for regenerating muscle damaged during injury or disease. Our current efforts are focused on determining what molecular signals and cell-cell interactions regulate satellite cells and regeneration. An additional question we have been exploring is the role of stem cells in maintaining myofibers, which are post-mitotic. Recently, we have found that satellite cells contribute to myofibers, even in the absence of injury and thus implicate satellite cells as playing a role in muscle homeostasis (Keefe, Lawson et al. 2015)
Muscle Connective Tissue and Fibrosis
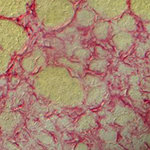 |
Transient fibrosis (red) surrounding muscle (yellow) during muscle regeneration |
Vertebrate muscle structure and function is critically dependent upon its connective tissue. The connective tissue ensheaths muscle and is important structurally for maintaining tissue architecture and functionally for transmitting muscle contractile force to adjoining tendon and bone. In addition, the connective tissue is an important component of the niche regulating muscle stem cells. However, connective tissue must be precisely regulated as connective tissue fibrosis, which is a feature of chronic injury, aging, and disease, can seriously impair muscle strength and elasticity.
We have identified the transcription factor Tcf4 (Tcf7L2) as a marker of the muscle connective tissue fibroblasts (Kardon et al. 2003, Mathew et al. 2011). We have generated (in collaboration with Melinda Angus-Hill and Mario Capecchi) two alleles of Tcf4 in mice, Tcf4GFPCre and a tamoxifen-inducible Tcf4CreERT, to genetically label and manipulate the connective tissue fibroblasts (Mathew et al. 2011, Murphy et al. 2011). Using this new molecular marker and genetic reagents, we are addressing multiple questions. What extracellular matrix components do the fibroblasts make? What triggers fibroblasts to a fibrotic state? How does fibrosis in muscle compare with fibrosis in other tissues?
Tissue Interactions During Limb Musculoskeletal Development and Congenital Limb Abnormalities
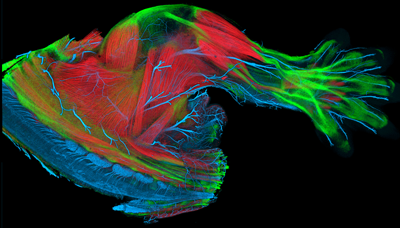 |
E14.5 mouse limb with muscle (red), tendon (green), and nerves (blue) labeled |
Development of the vertebrate musculoskeletal system requires the coordinated morphogenesis of muscle, muscle connective tissue, tendon, and skeleton. How muscles and tendons are patterned and assembled with muscle connective tissue is largely unknown and defective in multiple human genetic syndromes. Based on our previous work (Kardon et al. 2002, Kardon et al. 2003), we hypothesize that the muscle connective tissue and its interactions with muscle and tendon are critical for development of the musculoskeleton. To test this hypothesis, we are examining in mouse the morphogenesis of muscle, muscle connective tissue, tendons, and skeleton during limb development and when key genes are conditionally deleted. To visualize normal musculoskeletal morphogenesis, we are generating, in collaboration with Yong Wan and Chuck Hansen at The Scientific and Computing Institute, a 3-dimensional atlas of limb musculoskeletal development. This atlas will provide an integrative description of normal limb musculoskeletal development and will be a publicly available resource. A description of the methods employed to make this atlas, with some initial atlas images, are now published (Yong et al. 2012). This atlas will be an invaluable resource for evaluating mouse limb mutants. Our integrative analysis of multiple conditional limb mutants will elucidate general principles governing morphogenesis of the complex structure of the limb and provide insights into outstanding questions. How are multiple tissues, with different developmental origins, organized into the integrated structure of the limb? Are there particular processes or molecular pathways that are subject to failure and lead to congenital defects?
Tissue Interactions During Limb Muscle Regeneration
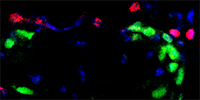 |
Pax7+ satellite cells (green) and Tcf4+ fibroblasts (red) |
Muscle regeneration requires the coordinated interaction of multiple cell types. In addition to the adult muscle stem cells (the satellite cells), muscle connective tissue fibroblasts are important regulators of muscle regeneration. To test the role of connective tissue, we used the tamoxifen-inducible Tcf4CreERT2 allele (which we generated in collaboration with the lab of Mario Capecchi) to genetically ablate connective tissue fibroblasts. Genetic ablation of MCT fibroblasts altered the dynamics of satellite cells, leading to premature satellite cells differentiation, depletion of the early pool of satellite cells, and smaller regenerated myofibers (Murphy et al. 2011). Thus we identified connective tissue fibroblasts as a novel and vital component of the niche regulating satellite cell expansion during regeneration. Identifying and testing the molecular signals from the connective tissue fibroblasts to the satellite cells is a major area of our current research.
Tissue Interactions During Development of the Diaphragm and Congenital Diaphragmatic Hernias
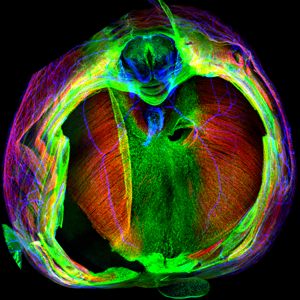 |
E14.5 mouse diaphragm with muscle (red), tendon (green), and nerves (blue) labeled |
The diaphragm is functionally the most important skeletal muscle in mammals, as it is essential for respiration. Strikingly, defects in diaphragm development are common birth defects (1:3000 births) that cause congenital diaphragmatic hernias (CDH) and result in high neonatal mortality. Given its functional importance and the frequency and severity of CDH, we focus on understanding the embryonic origins, cell-cell interactions, and genetic mechanisms underlying diaphragm development normally and during herniation. Similar to limb development, interactions between muscle and its connective tissue are critical for diaphragm development (Merrell et al. 2015). Furthermore, mutations in connective tissue are a potent source of CDH (Merrell et al. 2015). We are using a combination of in vitro and in vivo experiments to elucidate the genetic mechanisms and cellular processes regulating development of the diaphragm and CDH.
Evolution of the Diaphragm, a Novel Mammalian Muscle
Although the diaphragm is a functionally essential skeletal muscle in humans, the muscularized diaphragm is an evolutionary novelty that arose in and uniquely defines mammals. What genetic and developmental innovations led to this major mammalian innovation? The question of how morphologic novelties arise is a major question in evolutionary biology. While changes in tissue interactions and genetic architecture underlying the modification (e.g. addition of hand/feet to tetrapod limbs) or loss (e.g. pelvic armor reduction in stickleback fish) of structures are starting to be elucidated, considerably less is known about how complex morphological structures arise de novo. To discover what developmental innovations led to the evolution of the muscularized diaphragm, we are comparing the development of birds (chick), which represent an ancestral, non-muscularized state, with mice.

